Potassium sorbate is the potassium salt of sorbic acid; it is mass produced as a chemical additive in foods and drinks, in which it acts as a preservative. Potassium sorbate is especially good at preventing mould (fungal) growth.
How does potassium sorbate help to preserve food?
Potassium sorbate looks like a white salt and is highly soluble in water and ethanol. This solubility factor is important because potassium sorbate must dissolve in water to release its active from – sorbic acid. Sorbic acid is absorbed into fungal cells where it can either kill the cell or inhibit its growth; these are known as fungicidal and fungistatic activity, respectively. It also works to prevent bacterial growth. Potassium sorbate works best in acidic solutions around pH 4. However, adding sorbic acid directly to drinks or foods with a high-water content is not as effective as using potassium sorbate because sorbic acid is less soluble. Hence, you are more likely to find sorbic acid in cheese and dried fruit than in wines. Potassium sorbate is ubiquitous in wine, fruit juice and puree production and works best in acidic solutions around pH 4.
In the EU and UK potassium sorbate is listed as E202; sorbic acid is listed as E200
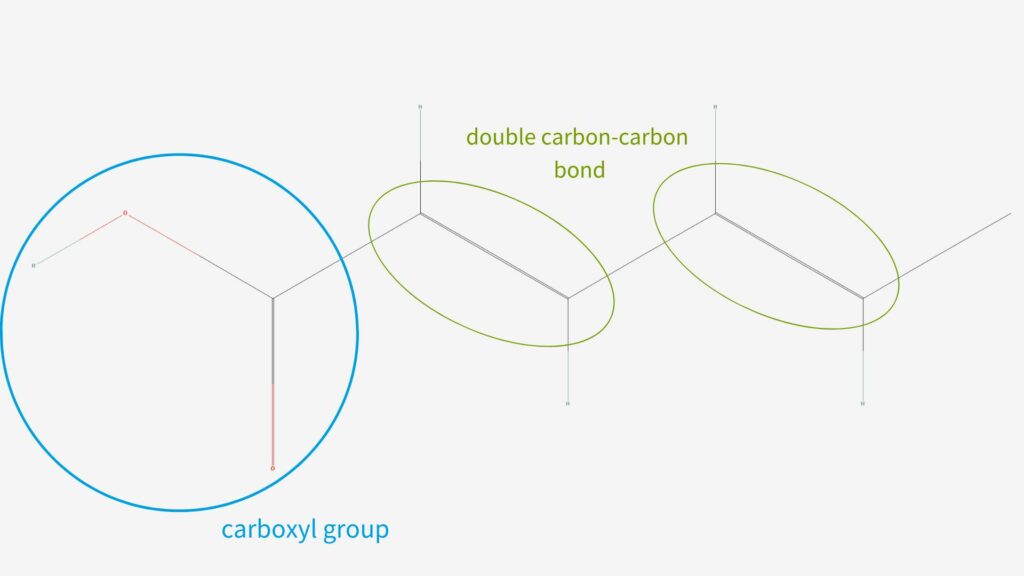
Chemical compositions of sorbic acid and potassium sorbate
Sorbic acid is a polyunsaturated carboxylic acid which means it has multiple double carbon-carbon bond attached to a carboxyl group. It is also a fatty acid, a group of carboxylic acids with atoms joined in straight chains. In the basic reaction that creates potassium sorbate, hydrogen ions in the carboxyl group are replaced by potassium ions from the potassium hydroxide molecules.
Since it is a sorbic acid salt, potassium sorbate has a similar structure but the oxygen-hydrogen bond in the carboxyl group is replaced by an oxygen-potassium bond.1
How humans discovered sorbic acid
Sorbic acid was first isolated from the rowanberry (Sorbus aucuparia), a fruit that grows on mountain-ash trees, in 1859 by German chemist Wilhem von Hofmann who managed to distil parasorbic acid from rowanberry oil, then hydrolysed parasorbic acid to produce sorbic acid.
In the 1980s sorbic acid took the place of nitrites as a preservative of meats. This came after discovering that nitrites are carcinogenic and cause botulism.
How is potassium sorbate made?
In the neutralisation reaction that creates potassium sorbate, hydrogen ions in the carboxyl group are replaced by potassium ions from the potassium hydroxide molecules.
- There are four key steps in producing potassium sorbate:
React ketene and 2-butenal to get the ester of sorbic acid at 30 – 80 °C (this is a condensation reaction)
- Cleave the ester of sorbic acid with water (hydrolysis) to isolate sorbic acid. To get a good yield of sorbic acid manufacturers will use an acidic catalyst like hydrochloric acid.
- Wash the solution with alcohol to remove waste products.
- Neutralise sorbic acid with potassium hydroxide to get a potassium sorbate solution.
- Dry out the solution by spinning at a high speed in a centrifuge; this produces potassium sorbate powder.2
Which countries export potassium sorbate?
China is currently the main exporter of potassium sorbate since its weather conditions are conducive to the mountain ash tree that contains the salt. Germany also exports but the unfavourable climate means that it produces less potassium sorbate. Therefore, China holds the market advantage and is able to increase the price of potassium sorbate; the nation has been encouraging more exports of the product because of rises in currency exchange rates and tax rebate increases.
Why do manufacturers use potassium sorbate and sorbic acid?
Different forms of potassium sorbate have different uses. The granular and powdered forms are essentially the same and used in cheese, wine, meat and dried fruits. While the crystalline form is used in processed fruits and vegetables, baked goods, and dairy.
Potassium sorbate and sorbic acid are favoured for their neutral taste and lack of odour which allow for use in various formulations. Plus, neither additive accumulates in the human body because they are used up in fatty acid metabolism.
At CZ we supply potassium sorbate that meets GFSI standards and aligns with the highest standards of food safety and good manufacturing practices. Our potassium sorbate is also HACCP and ISO certified, non-GMO and Halal, and is typically supplied from Asia and Europe in 25kg multiwall bags with other sizes also available. You can speak to a member of our Food Ingredients and Packaging team to find out more.
Potassium sorbate allergies
Rarely, some people have an allergic reaction when exposed to potassium sorbate. This typically presents as systemic contact dermatitis. To make food suitable for consumers with this allergy, manufacturer may opt for sodium benzoate (E211). However, food scientists have found an optimal ratio for combining potassium sorbate and sodium benzoate in food to reduce sensitivity to preservatives.
Alternatives to synthetic preservatives
Synthetic preservatives are popular in the industry because they are effective in small quantities and cheap. As more people adopt a plant-based diet, we are seeing rising demand for plant-based preservatives as well. Flavonoids are one such group. Flavonoids are a type of polyphenol found in fruits and vegetables like strawberries and kale. There is evidence that they work to inhibit bacterial growth, but evidence of anti-fungal activity is not as concrete, therefore it may be the best replacement for potassium sorbate. Although flavonoids and other polyphenols can be extracted from a wide range of plants, to become widely used in the food industry, they would need to be mass manufactured. For this to happen efficiently, manufacturers would need to opt for processes that involve modifying metabolic, and therefore genetic pathways, to get higher polyphenol yields.3
Did you find this helpful? Share it on LinkedIn, Facebook, or Twitter. Learn more about the products we source with these blogs.
Sources
- National Library of Medicine, Sorbic Acid. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Sorbic-acid
- Ningbo Wanglong Science and Technology Co., Ltd. Process for preparing potassium sorbate. Available at: https://patents.google.com/patent/CN1634844A/en
- Ofosu et al, 2020. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Available at: Frontiers | New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns (frontiersin.org)